Method Development and Validation of Lorazepam by Using RP-HPLC in Pharmaceutical Formulation
DOI:
https://doi.org/10.47070/ijraps.v7i4.139Abstract
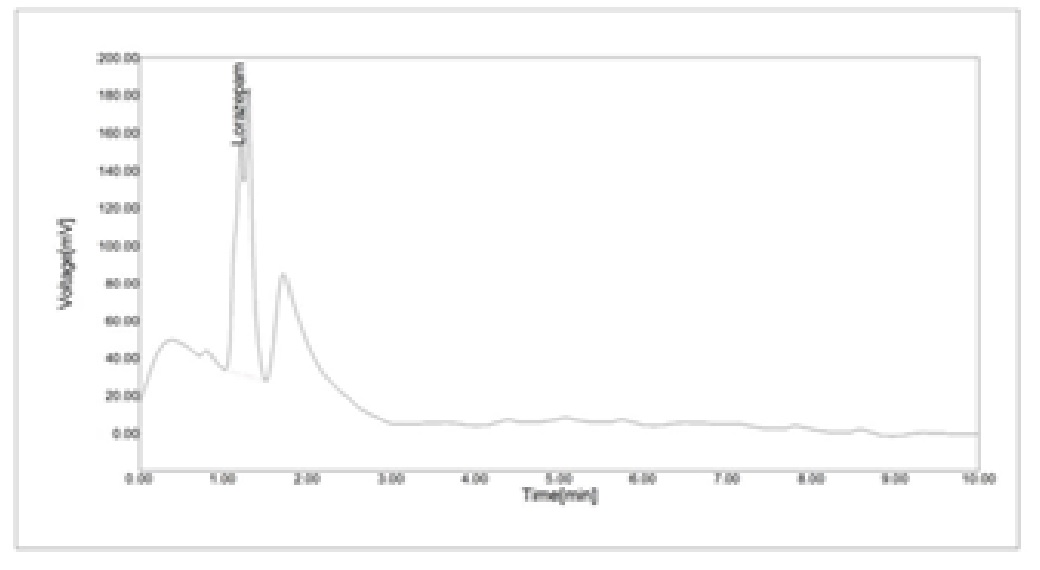
High pressure liquid chromatography (RP-HPLC) is a column chromatographic technique employing high pressure pump to pass both mobile phase and sample mixture through stationary phase column and perform efficient separation. A C-18 column with mobile phase containing Acetonitrile: methanol (80: 20) was used. The flow rate was 1.0ml/min. and were monitored at 210 nm. The retention time for Lorazepam was 1.3 min. The method was validated for linearity, accuracy, precision, limit of detection. The Mean percent recovery of Lorazepam from tablet formulation was found to be 94.80 %.
Downloads
Download data is not yet available.
Downloads
Published
2023-04-22
Issue
Section
Articles
How to Cite
1.
Method Development and Validation of Lorazepam by Using RP-HPLC in Pharmaceutical Formulation. IJRAPS [Internet]. 2023 Apr. 22 [cited 2026 Mar. 10];7(4):1-3. Available from: https://ijraps.in/index.php/ijraps/article/view/139

