Method Development and Validation of Latanoprost by using RP-HPLC in Pharmaceutical Formulations
DOI:
https://doi.org/10.47070/ijraps.v7i4.142Keywords:
Latanoprost, RP-HPLC, Precision, Validation.Abstract
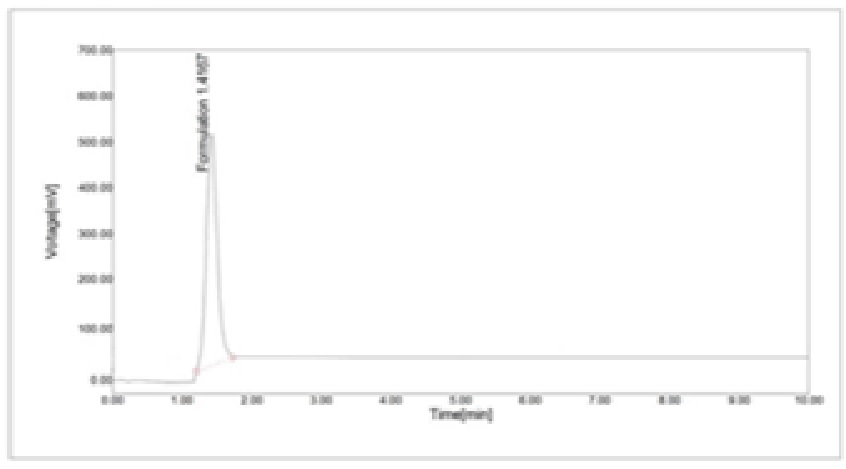
Chromatography was performed with a mobile phase containing a methanol of assay (99.8%) with flow rate of 1ml/min. Quantitation was accomplished with an internal standard method. The procedure was validated for linearity (correlation coefficient = 0.990), accuracy and Limit of detection (LOD) intraday precision. To test validation of the Latanoprost three factors were considered as linearity, precision, LOD where mobile phase, flowrate and pressure are respectively selected as methanol, 1 ml/min, 1600 pascals.
Downloads
Download data is not yet available.
Downloads
Published
22-04-2023
How to Cite
1.
K.Srinivas, D.Narendra, P.V.V.Varaprasad, G.Jaya durga madhuri, K.Eswari, N.Shanti priya. Method Development and Validation of Latanoprost by using RP-HPLC in Pharmaceutical Formulations. IJRAPS [Internet]. 2023 Apr. 22 [cited 2025 Jul. 6];7(4):14-6. Available from: https://ijraps.in/index.php/ijraps/article/view/142
Issue
Section
Articles


