Stability Indicating RP-HPLC Method Development and Validation for the Estimation of Ondansetron in Bulk and their Pharmaceutical Dosage Form
DOI:
https://doi.org/10.47070/ijraps.v5i7.118Keywords:
Ondansetron, Validation, HPLC, Dosage Form.Abstract
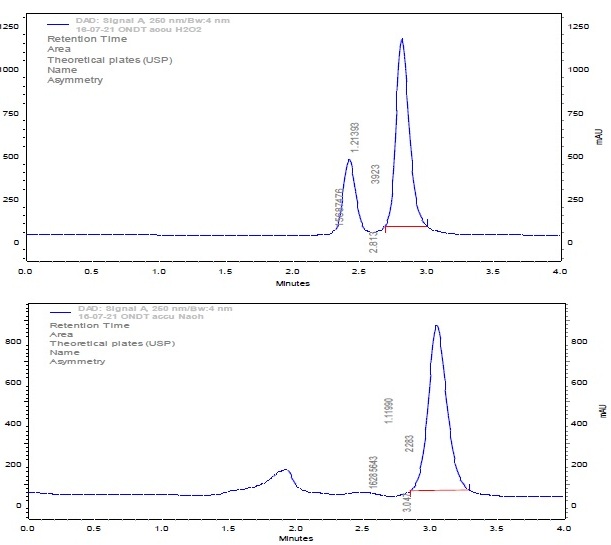
A simple, rapid, precise and accurate stability indicating RP-HPLC method was developed and validated for the estimation of Ondansetron in bulk drug and pharmaceutical dosage form. A Phenomenex C18 (150 mm × 4.6 mm I.D., 5 µm particle size) column was used as stationary phase with mobile phase consisting of 0.1% Formic acid (pH 4.25):Acetonitrile in the ratio of 50:50 V/V. The flow rate was maintained at 0.6 mL/min and effluents was monitored at 250 nm. The retention time was 2.91 min. The linearity of the method was observed in the concentration range of 5-25 µg/mL with correlation coefficient of 0.999. The method developed was validated for linearity, precision, accuracy, system suitability and forced degradation studies like acidic, alkaline, oxidative and hydrolytic stress conditions were performed as per ICH guidelines. The results obtained in the study were within the acceptable limits and hence this method can be used for the estimation of Ondansetron in pure drug and pharmaceutical dosage form.
Downloads